SELECTED IMPORTANT SAFETY INFORMATION: KOVALTRY®
is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, to any of the excipients, or to mouse or hamster proteins.
The Vial Adapter needleless reconstitution system and how to store KOVALTRY®
The Vial Adapter needleless reconstitution system contains1:
Vial Adapter with built-in 15-micrometer filter
2.5 mL or 5.0 mL prefilled diluent syringe
25-gauge butterfly needle

Storage at room temperature (up to 77°F) for up to 1 year1
Store KOVALTRY® at 36°F to 46°F for up to 30 months from the date of manufacture. Do not freeze. Within this period, KOVALTRY® may be stored for a period of up to 12 months at temperatures up to 77°F. Record the starting date of room temperature storage clearly on the unopened product carton. Once stored at room temperature, do not return the product to the refrigerator. The product then expires after storage at room temperature for 12 months, or after the expiration date on the product vial, whichever is earlier. Store vials in their original carton and protect them from extreme exposure to light.

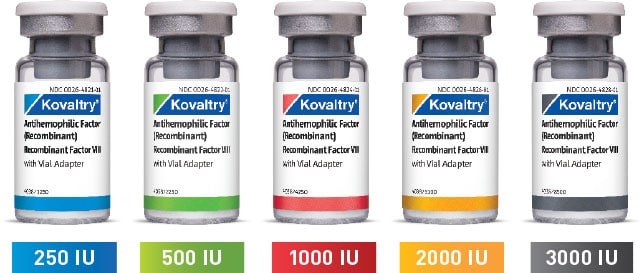
KOVALTRY® is available in a wide range of vial sizes1
Reconstitution with 2.5 mL and 5.0 mL diluent volumes
INDICATION FOR KOVALTRY®
KOVALTRY® Antihemophilic Factor (Recombinant) is a recombinant human DNA sequence derived, full length Factor VIII concentrate indicated for use in adults and children with hemophilia A for:
On-demand treatment and control of bleeding episodes
Perioperative management of bleeding
Routine prophylaxis to reduce the frequency of bleeding episodes
KOVALTRY is not indicated for the treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
KOVALTRY is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, to any of the excipients, or to mouse or hamster proteins.
Hypersensitivity reactions, including anaphylaxis, are possible with KOVALTRY. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. Discontinue KOVALTRY if symptoms occur and seek immediate emergency treatment.
KOVALTRY may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Neutralizing antibody (inhibitor) formation has occurred following administration of KOVALTRY. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all Factor VIII products. Carefully monitor patients for the development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor.
Hemophilic patients with cardiovascular risk factors or diseases may be at the same risk to develop cardiovascular events as non-hemophilic patients when clotting has been normalized by treatment with Factor VIII.
Catheter-related infections may occur when KOVALTRY is administered via central venous access devices (CVADs). These infections have not been associated with the product itself.
The most frequently reported adverse reactions in clinical trials (≥5%) were inhibitors in previously untreated patients (PUPs)/minimally treated patients (MTPs), and pyrexia, headache, and rash.
For additional important risk and use information, please see full Prescribing Information.
Reference: 1. KOVALTRY® [prescribing information]. Whippany, NJ: Bayer HealthCare LLC; 2021.



